Abstract
Background:
Anagrelide clinical trials in myeloproliferative neoplasms (MPN) were pioneered by the late Murray N. Silverstein M.D.(1928-1998) of the Mayo Clinic and the first publication involving 20 patients, including 17 with essential thrombocythemia (ET), appeared in 1988 (study launched in October 1985) (N Engl J Med 1988;318:1292). A larger study involving 577 patients, including 335 with ET, was subsequently published in 1992 and led to FDA approval in March 1997 (Am J Med 1992;92:69). Anagrelide has also been evaluated, in controlled studies, for its efficacy and safety as first-line therapy for ET; the results of these studies suggested that anagrelide was not inferior to hydroxyurea, in one study (Blood 2013;121:1720), but might have been harmful to patients in, the second study (N Engl J Med 2005;353:33); in the latter study, patients receiving anagrelide experienced higher incidences of arterial thrombosis, bleeding complications and fibrotic progression. These observations raised significant concern regarding an adverse survival impact of anagrelide in ET; unfortunately, the follow-up period of formal controlled studies were too short to accurately address the particular concern.
Methods:
The current study represents a retrospective examination of the potential impact of anagrelide therapy on survival and disease complication rates in ET. The study population was recruited from a consecutive cohort of adult patients (age ≥18 years) with MPN seen at the Mayo Clinic from 10/27/1967 through 12/29/2017. Diagnosis and determinations of fibrotic and/or leukemic transformations, were in strict accordance with the 2016 World Health Organization criteria (Blood 2016;127:2391). Statistical analyses were based on clinical and laboratory parameters obtained at the time of first referral to the Mayo Clinic which, in the majority of cases, coincided with initial diagnosis. All patients were followed from diagnosis until death or date of last follow-up or contact. Follow-up was until April 2018 and data collected via medical records or in certain cases, by directly contacting patients or their physicians. Survival and time-to-event curves were prepared using the Kaplan-Meier method and compared by the log-rank test. The JMP® Pro 13.0.0 software package was used for all analyses (SAS Institute, Cary, NC, USA).
Results:
1,076 patients with ET were considered (median age 58 years; females 63%); international prognostic scoring system for ET (IPSET; Blood 2012;120:1197) risk distribution was 28% high, 42% intermediate and 30% low; driver mutational status was JAK2 61%, CALR 25%, triple-negative 11% and MPL 3%. Comparison of cases diagnosed before (n=433) and after (n=643) the FDA approval date of anagrelide (1997) revealed the former cohort to be younger (median 57 vs 60 years; p=0.003), although the difference in IPSET risk distribution was not significant (p=0.14).
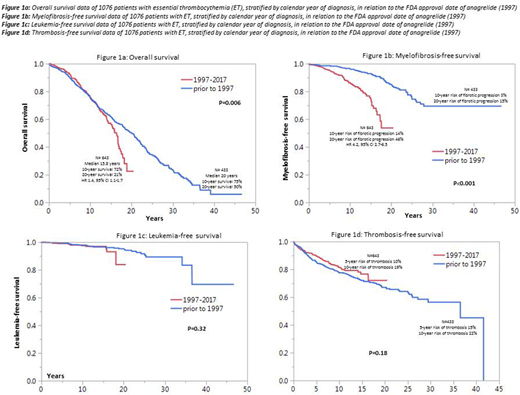
Figures 1a, 1b, 1c and 1d illustrate overall (OS), myelofibrosis-free (MFFS), leukemia-free (LFS) and thrombosis-free survival data comparing ET patients diagnosed before and after the 1997 FDA approval date for anagrelide; a significant difference was apparent for OS (p=0.006; HR 1.4, 95% CI 1.1-1.7) and MFFS (p<0.001; HR 4.2, 95% CI 2.7-6.5), in favor of patients diagnosed prior to 1997; during multivariable analysis that included IPSET and sex, the significant difference in both OS and MFFS were sustained; the difference in OS was most apparent after the first decade of diagnosis with 10- and 20-year survival rates of 75% and 50% for diagnosis prior to 1997 vs 72% and 21% for diagnosis between 1997 and 2017 (figure 1a); the 10- and 20-year risk of fibrotic progression was 3% and 15% for diagnosis prior to 1997 and 14% and 46% for diagnosis after 1997 (figure 1b); there was no impact on LFS (p=0.32; Figure 1c) or TFS (p=0.18; Figure 1d). Similarly stratified survival data in polycythemia vera (n=665) and primary myelofibrosis (n=1,282) showed no impact on survival (p=0.3 and 0.17, respectively).
Conclusions:
In a retrospective, but otherwise unbiased comparative analysis, we show significantly decreased OS and MFFS in ET patients diagnosed after the FDA approval date of anagrelide (1997); the increased risk of fibrotic progression noted is consistent with results of earlier prospective data (N Engl J Med 2005;353:33). Our observations regarding OS requires confirmation in a prospective controlled setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract